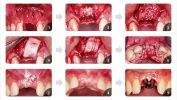
Product Description
Indications
– Burn and trauma
– Aesthetic plastic surgery
– Dermatology
– Obstetrics
– Gynecology
– Orthopedics
– Destistry
– General surgery: accidents, congenital diseases, periodontal disease,…
Product types
- SureDerm Graft
- SureDerm Implant
- SureDerm (dental)
Quality
- SureDerm becomes a part of the natural tissue of the patient and is integrated to become healthy tissue
- Antigens which cause cellular immune response have been removed so there are no rejection reactions inflammation with use
- There is no antigenic response so there is no need for skin testing
- The product can be folded or rolled to a desired thickness for use
- The rate of reabsorption is very low compared to autograft/xenografts
- The product has 15% elasticity so can be used on any part of the body
- The product maintains the 3-D structure of the skin and is highly biocompatible. It contracts minimally and has high elasticity and restructuring ability. A single surgery can be enough for all reconstruction
- No blisters or cysts form after transplantation
Safety
- Strict pathological and hematological testing of tissue donors for safety
- Each lot of product is produced from one donor
- Only the tissues approved by the AATB, EATB, KFDA are used
- E-Beam sterillization following final packaging
- In Vivo (animal testing) and In Vitro (laboratory) Testing
- No cases of viral infections have occurred so far
- SureDerm Safety Test
| HbsAg | Hepatitis B surface Antigen |
| HCV | Hepatitis C Virus |
| HTLV | Human T-cell Lymphotrophic Virus |
| HIV-1/2 | Human Immunodeficiency Virus |
| HIV-1 Ag | Human Immunodeficiency Virus |
| HIV by PCR | Human Immunodeficiency Virus by polymerase chain reaction |
| STS | Serelogic test for Syphilis |
| HBc | Hepatitis B core |
| CMV | Cytomegalo Virus |